Cancer Therapeutics CRC Deploys Surety’s AbsoluteProof
 Surety announced today that Cancer Therapeutics CRC Pty Ltd (CTx), an Australian pharmaceutical discovery and development company, has selected to use AbsoluteProof for its scientific IP protection.
Surety announced today that Cancer Therapeutics CRC Pty Ltd (CTx), an Australian pharmaceutical discovery and development company, has selected to use AbsoluteProof for its scientific IP protection.
Focused solely on developing newly discovered drug compounds from cancer research considered ‘high risk’ by bio-technology and pharmaceutical companies, CTx is required to meet strict electronic record protection standards and secure its data from unauthorized changes or tampering. AbsoluteProof’s independent, digital timestamping functionality meets these needs and continuously helps prevent intellectual property theft and manipulation, such as backdating “first-to-invent” status, around the clock. In addition, AbsoluteProof provides scientists from institutions around the world with a centralized, easily accessible system that allows them to focus on their research, rather than time-consuming administrative tasks, including the signing and witnessing processes that are common in the bio-pharma industry.
“We realize the value of our IP through licensing deals, so we need to demonstrate to any potential partner or licensee that all critical electronic records and files will withstand the most vigorous legal scrutiny,” said Dr. Tony Evans, CEO of Cancer Therapeutics CRC. “All archived content must be able to be confirmed independently, and there must be no possibility of collusion, forgery or backdating. We rely on the AbsoluteProof Service to provide us with that defence, and it has allowed us to focus on what really matters – developing drug solutions and saving lives.”
Read the press release here.
– Bob Flinton
VP of Marketing and Product Management
Surety, LLC
Surety Teams Up with SAFE-BioPharma Association to Provide Industry’s Strongest Digital IP Protection Service
 Today Surety announced that is has partnered with SAFE-BioPharma Association to combine AbsoluteProof with the SAFE-BioPharma digital identity management standard. This agreement will allow biopharmaceuticals to protect their IP and legally defend its authenticity throughout the chain of custody.
Today Surety announced that is has partnered with SAFE-BioPharma Association to combine AbsoluteProof with the SAFE-BioPharma digital identity management standard. This agreement will allow biopharmaceuticals to protect their IP and legally defend its authenticity throughout the chain of custody.
Under the terms of the agreement, Surety is able to incorporate the SAFE-BioPharma identity management standard – used to verify and manage digital identities and to apply digital signatures to electronic records – into products using its AbsoluteProof cryptographically based time-stamp service. The new alliance will jointly target laboratories in small and mid-size biopharmaceutical companies as they transition from paper-based to electronic lab management workflows and record-keeping processes.
“Our goal is to help improve R&D efficiencies across the industry by providing a simple, transparent and easy way for biopharmaceutical organizations to protect the ownership of their scientific intellectual property for the long term,” said Tom Klaff, CEO of Surety. “By not only signing their research content, but also ‘sealing’ it to lock down the time of creation, and offering a legal defensibility guarantee behind it, companies can be certain that their discoveries will have the strongest, most legally defensible ‘who’ and ‘when’ attributes associated with them for the life of their research.”
Read the press release here.
– Bob Flinton
VP of Marketing and Product Management
Surety, LLC
Tampering with History
 A Washington Post article recently revealed a case of data integrity manipulation, specifically dealing with a National Archive treasure. The artifact at hand was a recently discovered pardon issued to a Union solider by President Abraham Lincoln. The document, which was handwritten by the President and saved the solder from execution, was found 13 years ago by junior historians Thomas P. Lowry, and his wife, Beverly.
A Washington Post article recently revealed a case of data integrity manipulation, specifically dealing with a National Archive treasure. The artifact at hand was a recently discovered pardon issued to a Union solider by President Abraham Lincoln. The document, which was handwritten by the President and saved the solder from execution, was found 13 years ago by junior historians Thomas P. Lowry, and his wife, Beverly.
Putting aside the act of uncovering a historical artifact, the main attraction surrounding the document is specifically the date of signage. At first glance, experts believed that the document was created on the same day President Lincoln was assassinated (April 14, 1865), just hours before the shooting. However, as details surrounding the discovery unraveled, experts realized their assumptions were inaccurate.
While the President did in fact issue a pardon for the soldier, it was actually written on April 14, 1864, exactly one year before his death.
How exactly were the facts mixed up, especially considering the date was written directly on the pardon itself, you might ask? Simple. After growing suspicion about the darkness of the number 5 within the date, officials compared the document to that of a reprint from the 1950s, which in turn showed a different date, that of 1864 to be exact. As it turns out, a year’s worth of investigations led the National Archives to accuse Thomas Lowry of altering the document, basically to heighten the sensation related to its discovery. (A document written by Lincoln in the 1800’s is a great find, but one written on the same date and year as his murder, is a historian’s golden ticket.) Lowry denies any wrongdoing, but experts insist that in 1998, he transcribed a 5 over the 4 with a similar style pen that Lincoln used to handwrite the year 1864.
While finding and presenting a piece of history seldom seen by everyday citizens is newsworthy enough, what we’re really interested in is the tampering side of the story, and what we hope is an opportunity for the National Archives to evaluate the best practices they are using to ensure the authenticity of its records. While Lowry maintains his innocence, and the Archives has banned him from all facilities for life, the fact remains that the basic authenticity of Lincoln’s document was in question. The Archives was without a solution that could have easily, legally and verifiably proven the legitimacy of Lincoln’s document. If Archive officials had taken the necessary steps to put “time-of-creation” integrity controls in place, such as a digital scanning and “sealing” process, the alteration never would have been under investigation for the amount of time that it was. Instead, it would have been immediately clear that the pardon had indeed been subject to manipulation, and suspicion, rumors and arguments would have been put to rest.
We’re happy that a conclusion has been made in this case, and the tampering has been detected. (Though we’re sorry it ever happened at all.) We just hope it reminds people of all walks of business that any document or record can be tampered with, regardless if it was written by a President of the United States or not. And since this isn’t the first time historical documents have been messed with, maybe it’s time historians take a hard look at current data integrity protection standards.
– Bob Flinton
VP of Marketing and Product Management
Surety, LLC
Rescentris Integrates Surety’s AbsoluteProof into its Collaborative Electronic Research Framework (CERF) Solution
Surety announced today that Rescentris, an ELN and scientific content management software solution provider, has deployed AbsoluteProof into its CERF solution to strengthen the protection of its customers’ intellectual property.
Surety’s flagship product offering, AbsoluteProof, is a third-party, digital timestamping technology that delivers long-lasting protection; provides objective proof-of-record integrity and authenticity independent of an organization’s people, processes and systems; and is compliant with domestic and international timestamp standards. AbsoluteProof uses Surety’s patented hash-chain-linking methodology to provide ELN users with independent, irrefutable proof that their electronic scientific data has not been subject to forgery or compromise since the time it was created and “sealed.”
CERF ELN enables organizations to document the entire Research and Development (R&D) process from initial conceptualization through product development and production. Proof of the authenticity and integrity of the research data ensures the ability to have legally defensible records that enable laboratories to capitalize on their scientific intellectual assets.
Read the press release here.
Bob Flinton
VP of Marketing and Product Management
Surety, LLC
Surety’s Sales and Channel Teams Grow with Two New Hires
Surety announced today that it has expanded its team by hiring Dianne Lee Haas as Vice President of Channel Sales and Partnerships and Brandon Brown as Sales Manager.
Ms. Haas’ role at Surety will include the recruitment and management of Surety’s channel partners, and will be responsible for developing sales-generating strategies for strategic corporate programs. She has more than 25 years of experience in high tech business development, channel development and sales, and has worked with Fortune 50 companies to produce new revenue streams in both commercial and government sectors. Prior to her employment at Surety, Ms. Haas founded DLH Services, where she assisted technology companies in building alliances and partnerships to encourage overall revenue growth. She was also the Northeast Territory Manager at Intel where she was responsible for the development of business opportunities and the management of sales partner programs for the LANDesk enterprise management software products.
As Surety’s Sales Manager, Mr. Brown comes to Surety with more than 10 years of direct IT sales experience. In his new role, Mr. Brown will be accountable for Surety’s overall sales outreach efforts and revenue generation. His previous experience includes employment as an Inside Sales Manager at GTSI Corp., where he worked with government officials on state, local, Federal and channel levels.
Read the press release here.
Bob Flinton
VP of Marketing and Product Management
Surety, LLC
Upcoming Webinar: “How to Easily and Cost-Effectively Implement an Electronic ‘Sign & Witness’ Process to Protect Your Scientific IP For the Long-Term”
Join us on September 28 at 2:00 p.m. EDT as Surety partners with Pharmaceutical Technology to present the complimentary webinar, “How to Easily and Cost-Effectively Implement an Electronic ‘Sign & Witness’ Process to Protect Your Scientific IP For the Long-Term”.
Hosted by Bob Flinton, Vice President of Marketing and Product Management for Surety, the webinar will discuss the importance of protecting the integrity and legally defending the authenticity and ownership of scientific intellectual property (IP), data of which is critical to an R&D-dependent organization’s long-term success. Today, many bio-pharma organizations use manual “signing” and “witnessing” processes to position themselves to defend their valuable scientific IP, however many of these manual processes can be costly and time-consuming, as well as disruptive to research teams. During this webinar, Flinton will explore the cost-effective approaches for implementing efficient, electronic, time-stamped “Signing and Witnessing” processes that don’t sacrifice long-term IP protection, and enable scientists to focus on research instead of cumbersome administrative tasks.
What: Pharmaceutical Technology webinar, “How to Easily and Cost-Effectively Implement an Electronic ‘Sign & Witness’ Process to Protect Your Scientific IP For the Long-Term”
Key Learning Objectives:
- Understand the challenges inherent in protecting the integrity and legally defending the authenticity of scientific IP
- View how easily and quickly you could be up and running protecting your valuable scientific IP right at your desktop
- Discuss the relevance to your organization’s data authenticity and integrity protection needs
Who: Presenter: Bob Flinton, Vice President of Marketing and Product Management, Surety, LLC
Moderator: Jamie Carpenter, Multimedia Producer, Pharmaceutical Technology
Who Should Attend:
- Bio-informatics architects, IT security professionals
- Executives in pharma, biotech, academia and government sciences
- Legal, audit professionals of bio/pharma and biosciences organizations concerned with the long term protection of IP ownership
When: September 28, 2010 at 2:00 p.m. EDT
Registration: Register for free online

Surety announced today that Maquet SA has purchased Agilent’s OpenLAB ELN that includes Surety’s AbsoluteProof service. This partnership strengthens Maquet’s ability to secure the authenticity and ownership of its scientific IP.
Maquet, a major manufacturer of medical products, therapies and services for operating rooms and intensive care units, purchased the system to replace paper lab notebooks. To protect scientific intellectual property, Maquet personnel were regularly shuttling the notebooks to an off-site notary office to be manually time stamped. By implementing an automated digital notarization process, Maquet expects to streamline the efficiency and effectiveness of this process. The company says it was also seeking a tool to facilitate collaboration between research teams in France and Germany.
“OpenLAB ELN enables our customers to capture, manage and share their valuable research,” said Bruce von Herrmann, vice president and general manager, Agilent Software and Informatics. “The seamless integration of AbsoluteProof secures corporate intellectual property at the time of inception, automating the documentation of when patents are submitted.”
Read the press release here.
Bob Flinton
VP of Marketing and Product Management
Surety, LLC
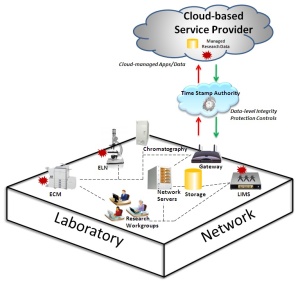 As more bio-technology and life sciences organizations look to reap the operational and cost-savings benefits that a transition to cloud computing can offer, the risk to their organization increases exponentially. The protection of critical scientific intellectual property (IP) as it leaves the perimeter of the development organization into the “hands” of a third-party custodian becomes paramount. Putting IP research “into the cloud” poses data integrity, authenticity and ownership concerns if essential security controls are not in place.
As more bio-technology and life sciences organizations look to reap the operational and cost-savings benefits that a transition to cloud computing can offer, the risk to their organization increases exponentially. The protection of critical scientific intellectual property (IP) as it leaves the perimeter of the development organization into the “hands” of a third-party custodian becomes paramount. Putting IP research “into the cloud” poses data integrity, authenticity and ownership concerns if essential security controls are not in place.
Executives of R&D-based organizations have a fiduciary responsibility to maximize and protect the value of their company’s assets. Intangible assets drive nearly 70 percent of a bio-technology company’s market value, predominantly in the form of scientific IP. There are documented cases in which a company’s loss of intellectual property, either to a competitor or to the public domain, has led to hundreds of millions of dollars in lost revenue, legal fees, reduced market valuations and damages related to class-action lawsuits.
Truly innovative life sciences and bio-technology companies making the move to the cloud take scientific intellectual property protection seriously, and integrate data-level security controls, such as trusted third-party timestamping, into their electronic lab informatics systems and workflows. They understand that litigation-readiness is a critical part of the IP protection process, and that having the power of proof is an essential safeguard in winning a future patent or legal challenge. Associated with cloud computing is the concept of “chain-of-custody” and being able to legally defend “time-of-creation” and ownership of scientific intellectual property as it changes hands is of utmost importance.
As R&D organizations move to the cloud, they should mitigate the risk of losing scientific intellectual property. The best way to mitigate this risk is to put in place security controls that enable the company to validate the integrity and prove the authenticity of the electronic lab data it needs to support its scientific intellectual property claims. Such controls can prevent these files from being successfully challenged and eliminated as evidence, and can stand up and successfully be defended in terms of “ownership.” For the cloud services provider, integrating these data integrity protection controls, as well as a legally defensible “guarantee” into its own cloud service platform can offer key competitive differentiators over other providers in the market.
Surety’s AbsoluteProof is a third-party, trusted timestamping solution that when integrated into a company’s electronic lab informatics management processes or into a cloud service provider’s platform, enables the organization to prove, independently of their people, processes and systems that the scientific records have never been manipulated since the time they were created, “signed and sealed.” Surety is the only trusted timestamp authority that guarantees the legal defensibility of its service.
– Bob Flinton, Vice President of Marketing and Product Management, Surety
On the Road with Surety
 Surety LLC, along with Adobe Systems, Inc., Docusign, Inc., Simplifile, LC and Tru-Dataintegrity, will be presenting its electronic record integrity protection solutions at the 2010 Uniform Law Commission Annual Meeting in Chicago this weekend. The Electronic Technology Demonstrations event will take place on July 10 from 8:00 a.m. – 5:00 p.m. at the Palmer House Hilton. Surety executives will be on hand throughout the day to discuss with attendees the importance of electronic data integrity protection and demonstrate to you the ways in which Surety’s “Sign and Seal” digital timestamping tools are able to protect, defend and authenticate electronic records and other critical intellectual property files.
Surety LLC, along with Adobe Systems, Inc., Docusign, Inc., Simplifile, LC and Tru-Dataintegrity, will be presenting its electronic record integrity protection solutions at the 2010 Uniform Law Commission Annual Meeting in Chicago this weekend. The Electronic Technology Demonstrations event will take place on July 10 from 8:00 a.m. – 5:00 p.m. at the Palmer House Hilton. Surety executives will be on hand throughout the day to discuss with attendees the importance of electronic data integrity protection and demonstrate to you the ways in which Surety’s “Sign and Seal” digital timestamping tools are able to protect, defend and authenticate electronic records and other critical intellectual property files.
For more information about the event, visit www.nccusl.org. If you are interested in learning more about digital timestamping tools and how they can record the exact date, time and form in which an electronic document existed in, feel free to let us know. We’ll be happy to have a conversation with you about IP and electronic records protection and the tools you need to secure your most valuable electronic assets.
We look forward to meeting with you!
Upcoming Events
The summertime brings about travel, and that’s just what we here at Surety will be doing this summer. Our executives will be hitting the road and attending a variety of biotech events, addressing topics ranging from pharmaceutical technologies to manufacturing techniques. Take a look at your schedule and see if you can meet up with us. We’d love to see you out and about and discuss with us the importance of data integrity solutions and best practices for intellectual property protection.
Global Pharma Manufacturing Summit
Boston, MA
June 14 – 15, 2010
Pharma Innovations Summit
Philadelphia, PA
July 27 – 28, 2010
